Skip to content
TMB – for immunotherapy decisions
Tumor Mutational Burden (TMB) is an emerging, independent predictive biomarker of immunotherapies in multiple tumor types, including lung cancer. TMB is an FDA approved biomarker for immunotherapy decisions.
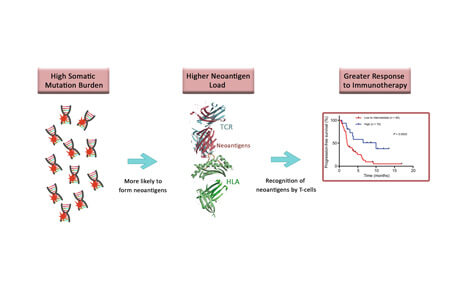
TMB refers to the total number of somatic mutations that exist within a tumor’s genome.
A subset of these mutations may result in expressed proteins, termed neo-antigens that are not recognized by the host’s immune system as self, and therefore have the potential to be immunogenic, leading to an anti-tumor immune-mediated response.
Tumors with a high mutation burden may have a higher rate of neo-antigens which, in principle, would be expected to be more immunogenic (and consequently more responsive to immunotherapy) than tumors with comparatively low mutation burden.
Results report of prime DX molecular cancer test include TMB rates.
2020 Genekor Medical S.A.. All Rights Reserved.
Web Design 

We use cookies that are absolutely necessary for the operation of this website and the provision of functions that you requested. These cookies remain enabled throughout your visit. Analysis cookies are installed in order to enhance your overall experience of browsing the site, to measure our audience, to collect useful information that will allow us to offer you information tailored to your interests. These cookies will be activated only if you give us your consent. By clicking "Accept " you give your consent to the use of analytics cookies; by clicking on "REJECT " or by continuing to browse the website without any choice, only the technically necessary cookies will be stored in your browser. Learn more about cookies at our Cookies Policy and click on Cookie settings to set and / or modify your cookie preferences. In case you would like to be informed about the use of cookies please contact the following email: dataprotection@genekor.com ACCEPT REJECT Privacy & Cookies Policy