PHYSICIANS’ FAQs
Any patient with a solid tumor is eligible for the PrIME test
prime DX test results include a thorough explanation of all the findings, such as the following information.
- Molecular profiling of the tumor tissue with details on specific gene mutations with interactive feed of any related information about that specific
- Suggested on-label Therapies that can be of great benefit to the specific patient with interactive feed of clinical trials
- Suggested off-label therapies that can be of great benefit to the specific patient with interactive feed of clinical trials
- Suggested investigational therapies with interactive feed of clinical trials
- Treatments with associated resistance- that will not be of benefit to the specific patient
- Medication suggestions with drug recorded indications
15 Working Days
For prime DX Assay we need a paraffin block that contains the tumor tissue. We need one paraffin block or four unstained paraffin sections of 3μm: in positive charged slides (air dried, not baked), placed on top of the slide, without folds and six unstained slides of 10μm.
For the prime DX Liquid analysis we need blood in special vials that you will receive from Genekor. (Cell-Free DNA BCT®(10ml) and Cell-Free RNA BCT®(10ml)).
- Quantity and quality of information
- Reduced time
- Reduced costs
As more targeted therapies aim at the gene changes detected in a small number of patients, a multi-gene panel results in the production of large volumes of multilevel information useful for individualized treatment of patients. Thus, it increases the likelihood of finding a therapeutic target for a patient with on-label treatment, off-label treatment and/or participation in clinical trials, using only a small sample of tissue and analyzing several genes simultaneously, rapidly and at low cost.
It is known that medication that is related to specific biomarkers and is indicated for one type of cancer may also benefit patients who have the same alteration in the same biomarker but in another type of cancer. Access to off-label drugs varies depending on the type of cancer, the biomarker and the data currently available on the specific drug. This also often determines the coverage of the off-label drug by the insurance company.
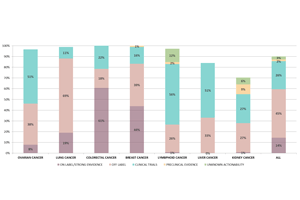 Interactive Results Report: The results report incorporates interactive links, which redirect the physician to information about the specific mutation of the gene, clinical trials for on-label & off-label treatments, and all the ongoing clinical trials connected, offering a continuous, up-to-date & clear picture to the physician and helping in the final choice of personalized treatment.
Interactive Results Report: The results report incorporates interactive links, which redirect the physician to information about the specific mutation of the gene, clinical trials for on-label & off-label treatments, and all the ongoing clinical trials connected, offering a continuous, up-to-date & clear picture to the physician and helping in the final choice of personalized treatment.
Continuous support to the doctor and the patient from our experienced team: Accessibility to scientific and procedural support, as well as information, is extremely important in our field. We know this and provide support to physicians and patients through the customer service center and through our scientific advisors, who are knowledgeable and specialized in order to constantly support the work of physicians in the best possible way.
More useful information than any other similar test: BBy analyzing 1021 unique cancer genes and the immunotherapy biomarkers HLA, MSI, TMB and PD-L1 as well as the LOH biomarker for PARP inhibitor therapy, the prime DX test the prime test gives access to the largest volume of usable information to date, thus providing the clearest and most reliable picture to the physician than any other similar test.
In cases where there is not enough tissue or the existing tissue does not have good quality DNA and / or RNA, it is recommenced the prime DX Liquid
The new prime DX Liquid test also consists of a panel of 1021 genes, which analyzes the two immunotherapy biomarkers MSI and TMB. It also includes Loss of Heterozygosity (LOH) analysis, which can be used as a biomarker for PARP inhibitor therapy. This enables the physician to design an effective treatment for the patient, including immunotherapy, chemotherapy, PARP inhibitors, and the patient's participation in clinical studies when required.
The PrIMe results report is intentionally designed to have a comprehensive approach to all treatment options available to the physician and the patient, enabling them to individualize disease management. One of these options is clinical trials, the importance of which is judged on a case-by-case basis.


